Medscape, August, 2023: John M. Mandrola, MD, has raised the question whether the doctors should really follow the cardiology based published evidence in medical journals evolving from observational studies. Robert Yeh, MD, from Beth Israel in Boston has emphasized in his publication ‘Bringing the Credibility Revolution to Observational Research in […]
Eli lily’s drug passes 2nd trial with flying colors targeted to reduce obesity
Shots, Health News from NPR, August 21, 2023: Ozempic and Wegovy, the diabetes and weight loss drugs created a buzz in the summers of 2022. Two clinical trials result on similar medication by pharmaceutical giant Eli Lilly astonished the doctors. Tirzepatide, traded as Mounjaro, specific for diabetes, eventually showed higher […]
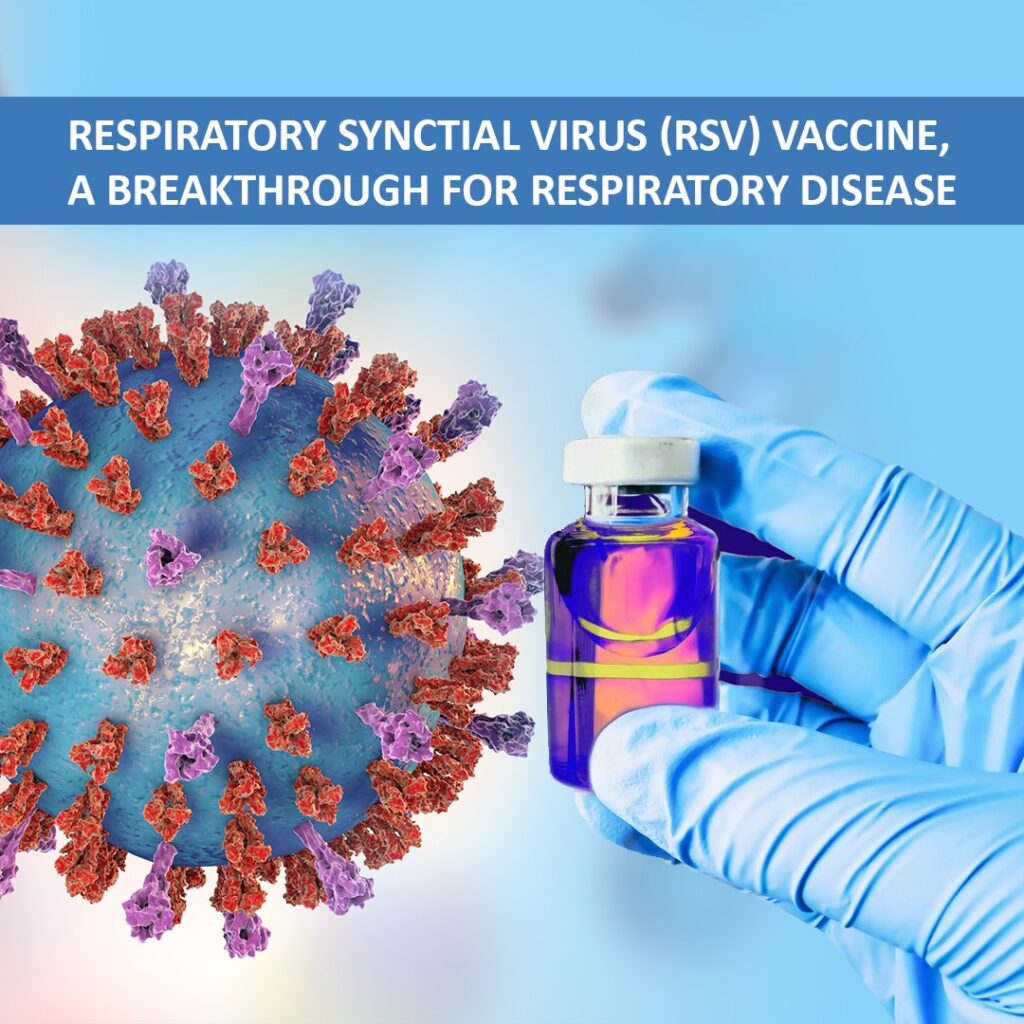
Respiratory syncytial virus (RSV) vaccine debut after 50 years
The New York Times, August 21, 2023: After 50 years of repeated failed attempts of discovering respiratory syncytial virus (RSV) vaccine, The U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention has approved Pfizer respiratory syncytial virus (RSV) vaccine – ABRYSVO™ for older adults, making […]
Abbott India recalls Digene gel batches following DCGI alert
The Times of India, New Delhi, September, 2023: Abbott India has initiated a recall of Digene gel due to taste and odor complaints, following a warning alert from the Drug Controller General of India (DCGI). While Digene is known for its pink color and sweet taste, the recalled product was […]
Scrub Typhus: A Monsoon Season Threat in India
The Indian Express, New Delhi, September, 2023: Scrub typhus, a concerning bacterial infection caused by Orientia tsutsugamushi, is claiming lives in Rajasthan and Himachal Pradesh. Over 700 cases have been reported, with at least five fatalities. This infectious disease spreads through mite (chigger) bites in lush, vegetated regions during the […]
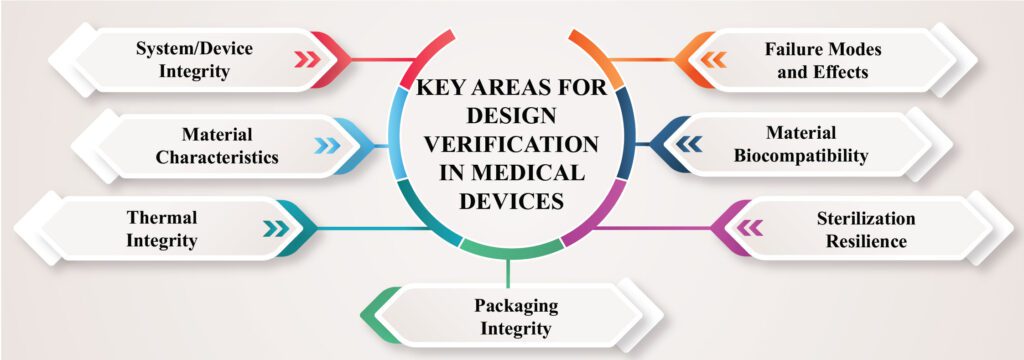
Medical Device Development : The Crucial Role of Claim Validation
Medical Device Design and Development In the process of developing and introducing new medical devices to the market, verifying the essential design features is crucial. Design verification, which involves testing, inspection, and analysis, is a necessary step to ensure compliance with quality management standards such as ISO 13485. Additionally, validating […]
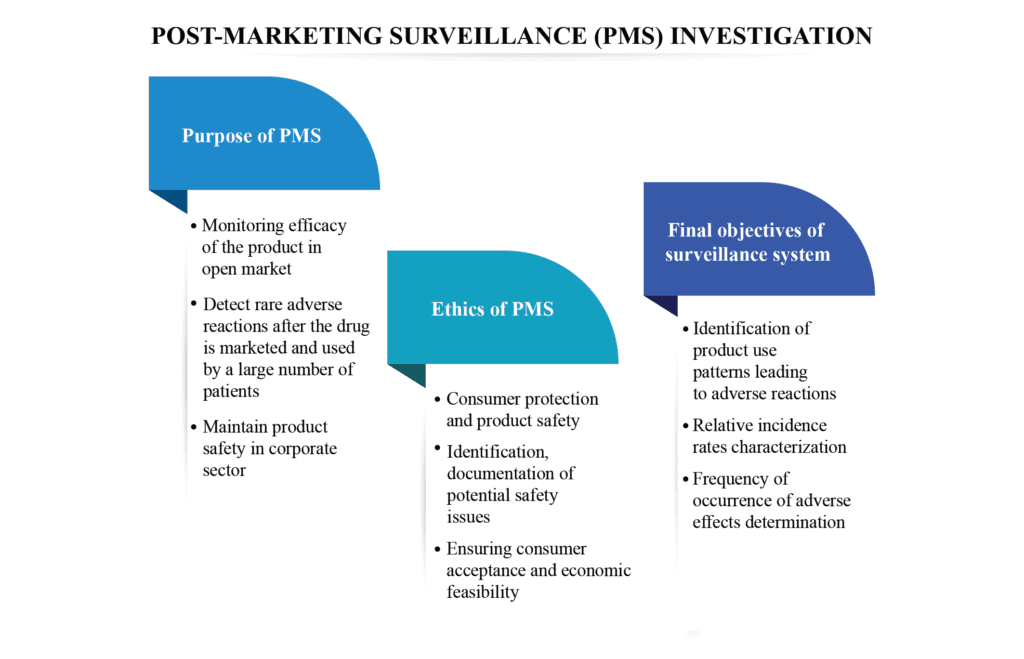
Post Marketing Surveillance : Ensuring Patient Safety beyond Clinical Trials
Post marketing surveillance (PMS) refers to monitoring of device/drug performance after successful completion of the clinical studies and the implementation of suitable action to improve patient safety. It is conducted after product is approved for sale by the European Union Medical Device Regulation (EUMDR) and the United States (US). It […]
Clinical Trials – Latest Technologies and Current Trends
Clinical Trials – Massive innovation was seen in clinical trials in the past couple of years, abating the fear of COVID-19 spread. Countless benefits from finding preventive measures, therapeutic drugs to developing vaccines have given the industry a more prominent audacity to take larger challenges. Not only the doctors and […]
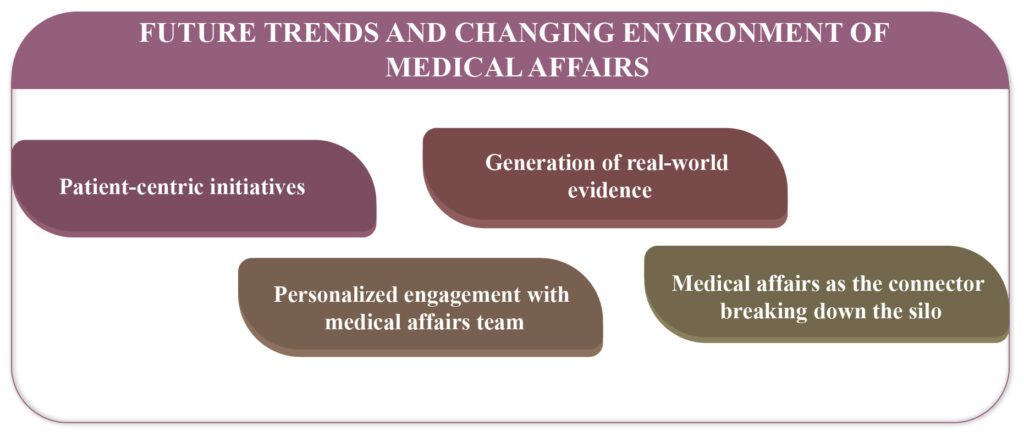
Medical Affairs – Future trends and changing environment
Medical Affairs (MA) focuses to find innovative solutions and continues to evolve fulfilling the needs of all healthcare providers and patients. Following recent trends have emerged in the life science industry. Patient-Centric Initiatives Emphasized patient-centricity that involves engaging the patient population, collaborating, and creating initiatives to satisfy the requirements of […]
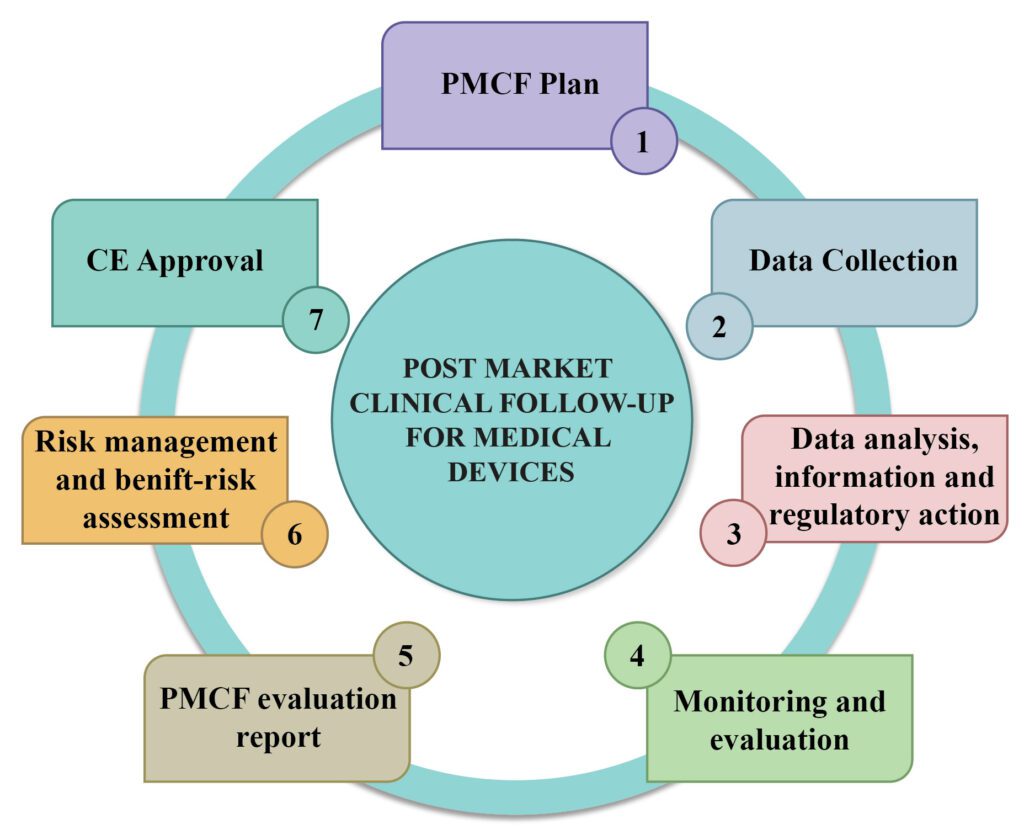
Post market clinical follow-up (PMCF) – An essentiality for Medical Devices
Post market clinical follow-up and Post-market surveillance (PMS) refers to the monitoring of medical devices after they have been cleared for sale and are in use by the public. One of the several types of post-marketing surveillance on the use of medical devices stipulated by the European Union Medical Device […]
Recent Posts
- Regulatory Affairs in Action: Recent FDA Drug Approvals and Medical Breakthroughs
- Medical Writing Regulatory for Product Excellence: Crafting the Path to Success
- CLINICAL DATA MANAGEMENT PROCESS OVERVIEW
- Clinical Trial in India: Exploring Innovative Therapies
- Healthcare Survey Role in Enhanced Journey: Personalized Care at its Best