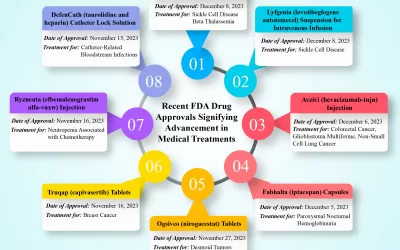
Clinical Trials – Latest Technologies and Current Trends
Clinical Trials – Massive innovation was seen in clinical trials in the past couple of years, abating the fear of COVID-19 spread. Countless benefits from finding preventive measures, therapeutic drugs to developing vaccines have given the industry a more prominent audacity to take larger challenges. Not only the doctors and researchers, but patients have also developed a distinct shift in the values of self awareness of their overall health and well-being, including their medical treatments. Industrial reconstruction will continue to evolve in 2023, as the research professional have drilled deeper to identify operational possibilities.
1. Adapting Decentralization Methods
Decentralized clinical trials (DCTs) have been at an upper hand over the last years, with increased acceptances from the investigators and sponsors. DCTs have assisted in overcoming huge obstructions like patient recruitment. The rapid change seen in the digital innovations is still coping up with the technological gap and needs further support. The rate of use of decentralization method, suggests that, additional 24% trials are expected to be decentralized by 2024.
Patient satisfaction and clinical site staff training are the two major factors in the fabrication of DCTs models. It is definitely proven that the patient recruitment process in decentralized method is easier compared to the traditional method, but it also plots a challenge of having only a group of participants who are technically literate. In other words, the success of trial related recruitment depends on easily accessible information and instructions to the population.
Advanced technologies, like wearable devices help in passive data collections during trials. These are also helpful in collecting mass data over time, improvising the source accuracy and pacing up the trial processes. The sponsors, in 2023, will have to put efforts in delivering DCT set ups that matches the patient needs, builds trust and shortens drop outs.
2. Artificial Intelligence (AI) and Machine learning (ML)
Integration of AI and ML are seen making huge impact on research and pharmaceutical companies. AI and ML provide endless opportunities in clinical trials and healthcare industries. They add value throughout the spectrum of clinical trials, from drug discovery to pre-trial planning through trial execution to data analysis. For example, using pattern recognition, ML can be used for X-Rays and MRI scans, to find patterns indicating particular disease. Some benefits of AI and ML are jotted below:
- Automation of various trial processes reduces staff workload and minimizes errors.
- ML helps in screening subject participants, predict data analytics and also forecast potential to avoid emergencies.
- Protocols and study designs are optimized to be even more patient centered, improving patient experiences, preventing dropouts,
- A one entry push in a system can update data into all connected systems, which can ease data entry, minimizing duplicate entries and saving time.
3. Clinical Trial using Smart devices
There is an enormous growth in the use of smart watches, smart phones, pulse oximeters, chest straps, apps, other monitoring sensors etc. now days. These devices track user’s physical activity using smart and/or GPS technology that are normally not possible to capture during onsite visits. They measure essential health data such as heart rate, respiratory rate, blood oxygen saturation level, sleep pattern, step counts and body posture. These devices are guiding the DCTs by recording remote data from the subjects in many therapeutic areas like asthma, cancer, schizophrenia, diabetes etc…
Major benefits include
- Predicting analytics early can help in patient retention, by checking for risks and taking immediate action.
- Prompt communication leads to better subject retention, encouraging active participation.
- Trials expenses are reduced due to decreased time and expense of clinic visits.
4. Clinical Trial Data Management
With time as the clinical trials and their source data are increasing in volume and complexity, their management becomes crucial and has led to discovery of interesting data management tools. To identify suitable subjects for clinical trials, the healthcare and research organizations are analyzing electronic health records (EHRs). To gather data from varied populations, the principal product designing team applies data analytics, to explore new and efficient treatment for underprivileged groups. Electronic case report form (eCRF) and electronic patient outcome (ePRO) are types of electronic tools for enhancing subject participation processes.
In cloud-enabled electronic data capture, research teams can securely store large data from multiple sites. It also offers expedited clinical trial operations on various patients and regions. Benefits like cloud computing, real time data transfer and feedback, data fixing are a great advantage for contract research organizations in conducting fast, structured and cost-effective clinical trials.
WorkSure® has an eminent team of experts, providing solutions that fairly improve the quality, feasibility and efficiency of clinical research. We provide biopharmaceutical companies, institutions with new and advanced treatments to patients, while maintaining the highest standard of participant protection. By setting up high quality DCTs, we have implemented better screening methods, while minimizing patient dropouts. We have also incorporated real-world insights for recruiting patients relevant to trials.