Clinical Trial in Nutraceutical World: Unveiling the Science behind Wellness
Clinical Trial in Nutraceuticals, which combine nutrition and pharmaceutical properties, have gained significant attention in promoting physical health, immunity, and longevity. A nutraceutical is any substance that is a food or part of a food that provides medical or health benefits, in addition to its basic nutritional value. These products play a crucial role in maintaining normal physiological functions and protecting against chronic diseases. With changing lifestyles and evolving health trends, the global nutraceutical market has experienced substantial growth. To demonstrate unique health benefits and prove the efficacy of their nutraceutical products, companies are increasingly turning to nutraceutical clinical trials.
Reasons Why Nutraceuticals are Gaining Traction Today:
Nutraceutical products are highly valued for their antioxidant properties and their potential to prevent life-threatening diseases. Manufacturers in the nutraceutical and food ingredient industry are actively seeking to market their products by highlighting their unique health benefits. As competition increases, companies are developing unique selling propositions (USPs) to differentiate themselves, leading to a surge in demand for nutraceutical trials.
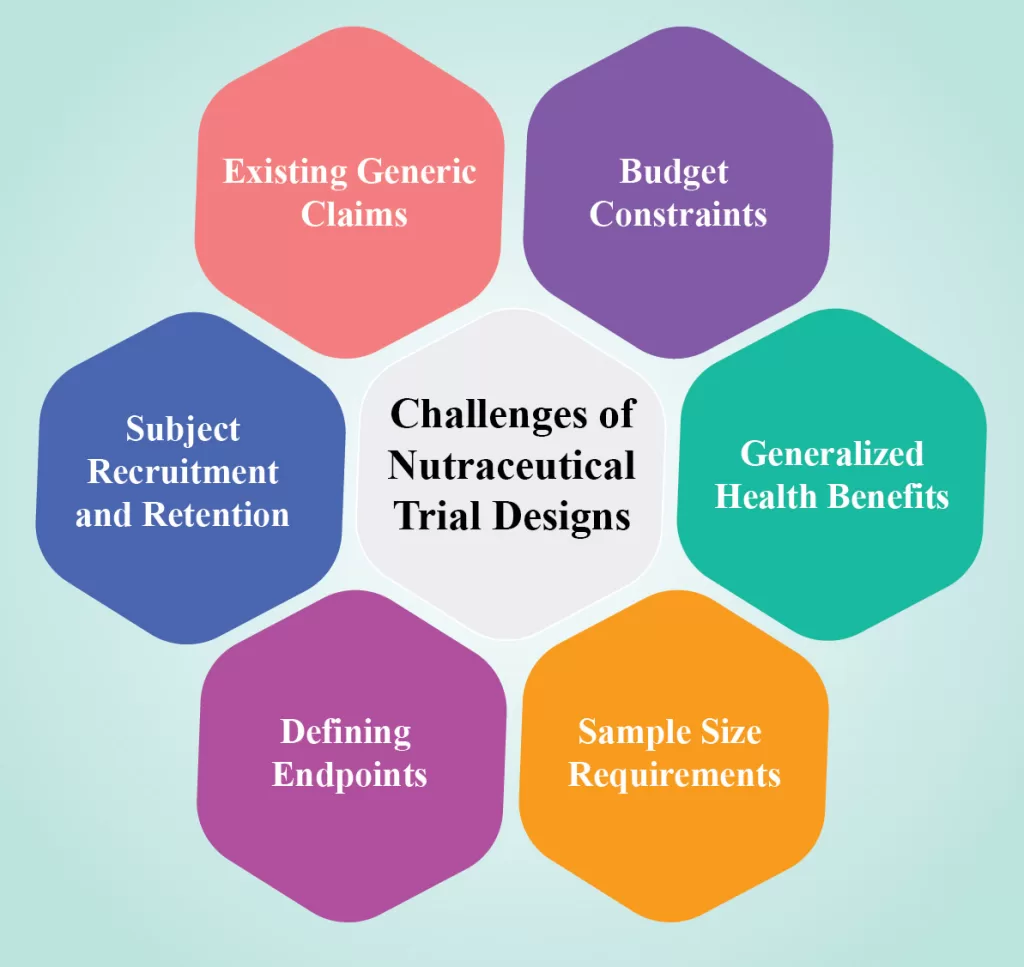
Challenges of Nutraceutical Trial Designs:
Designing nutraceutical trials poses several unique challenges for researchers. Many companies in this sector are unfamiliar with clinical trial designs due to various reasons.
- Existing Generic Claims: Generic claims related to vitamin and mineral content already exist and may limit the incentive for further research.
- Budget Constraints: Food and ingredient manufacturers often allocate limited budgets to trials, making it challenging to conduct comprehensive studies.
- Generalized Health Benefits: Nutraceuticals generally offer milder and more generalized health benefits compared to pharmaceutical drugs. Unlike pharmaceuticals, which undergo targeted testing for specific medical conditions, nutraceuticals are often marketed for overall well-being. Consequently, their health effects may be less potent and subject to fewer comprehensive investigations in rigorous clinical trials.
- Sample Size Requirements: Larger sample sizes are necessary for nutraceutical trials compared to conventional drug trials. This increased need for participants can lead to higher costs, longer recruitment periods, and potential difficulties in finding a diverse and representative study population, posing a considerable challenge for conducting nutraceutical clinical trials.
- Defining Endpoints: Unlike pharmaceutical trials with well-defined disease targets and mechanisms of action, nutraceuticals often have complex interactions and broader health claims. Selecting appropriate endpoints that reflect the intended effects of the product and establishing clear, evidence-based claims can be intricate. Conducting pilot studies to gain insights incur additional costs.
- Subject Recruitment and Retention: Recruiting subjects for nutraceutical trials is challenging, as participants must maintain a healthy lifestyle and provide comprehensive information. Dropout rates are typically higher compared to pharmaceutical trials.
Strategies for Effective Nutraceutical Trial Design:
Despite the complex challenges, nutraceutical trial designs can be managed effectively through structured approaches.
- Surrogate Markers: Using surrogate markers can help establish the preventive qualities of a product within a reasonable timeframe. Surrogate markers should be reliable and acknowledged by consumers. For example, rather than making a direct claim of a product “lowers the risk of strokes or heart attacks”, an alternative approach would be to make a surrogate claim that it “decreases levels of bad cholesterol linked to an elevated risk of strokes and heart attacks”.
- Blinded Trials: Double-blinded trials with placebos are the gold standard, minimizing bias and maintaining the integrity of the study. Open-label trials may be considered when designing suitable placebos is challenging.
- Controlled Trials: Incorporating control groups in trials allow comparison between nutraceuticals and placebos, providing a better understanding of the observed changes in subjects.
- Inclusion Criteria: Acknowledging lifestyle variables and implementing strict participant inclusion and exclusion criteria are crucial in nutraceutical trials. This ensures that the effects of the nutraceuticals can be observed and evidenced in the selected subjects.
- Generable Data: Trial data should be representative of a wider population, striking a balance between homogeneity and diversity. Running trials at multiple centers captures diverse demographics and environmental factors, enhancing data consistency and reliability.
Conclusion
Nutraceuticals are gaining traction as consumers seek products that offer both nutritional and medicinal value. To prove the health benefits of these products, companies are increasingly relying on nutraceutical clinical trials. Despite the challenges associated with trial design, strategies such as using surrogate markers, implementing controlled and blinded trials, considering inclusion criteria, and generating representative data can enhance the effectiveness of nutraceutical trials.
At WorkSure®, we specialize in conducting nutraceutical clinical trial, offering end-to-end services from study design to commercialization. Our experienced teams ensure appropriate recruitment, sample, and data handling, along with providing protocol planning and data analysis. We provide comprehensive services to support clients throughout the nutraceutical trial process, ensuring reliable results and valuable insights for the development of evidence-based nutraceutical products.