Medical Device Development : The Crucial Role of Claim Validation
Medical Device Design and Development
In the process of developing and introducing new medical devices to the market, verifying the essential design features is crucial. Design verification, which involves testing, inspection, and analysis, is a necessary step to ensure compliance with quality management standards such as ISO 13485. Additionally, validating various characteristics of medical devices and their materials can help identify potential issues that may impact regulatory approval or consumer acceptance. Lets understand the significance of claim validation studies during the product development process and highlight specific design aspects that greatly benefit from verification assessments.
The Role of Control Processes in Medical Device Design and Development
To meet the United States Food and Drug Administration (FDA) regulations, medical device manufacturers must establish procedures to control the design of their devices as part of their quality management system. A robust design control process not only fulfills quality management requirements but also facilitates efficient and resilient product design and development. By identifying and addressing issues early in the process, the overall timeline for the development of medical devices is optimized. Moreover, an effective design control process ensures that the final medical device is suitable for its intended use and poses no unforeseen risks to patients.
Understanding Design Verification
Design verification is an integral part of the product design control process. Its purpose is to confirm that the designed medical device meets the original requirements and specifications. It involves various techniques such as physical inspections, performance analysis, and testing to establish compliance with design input. Depending on the complexity of the device, the necessary level of design verification can be achieved by utilizing verification techniques either individually or in combination.
Differentiating Verification and Validation
Verification and validation are separate but complementary activities in the product design control process. While verification confirms that the device meets the design requirements, validation involves confirming, through objective evidence, that the device fulfills its intended needs and uses. Verification ensures compliance throughout the design and development process, while validation assesses the device’s overall performance once it is produced.
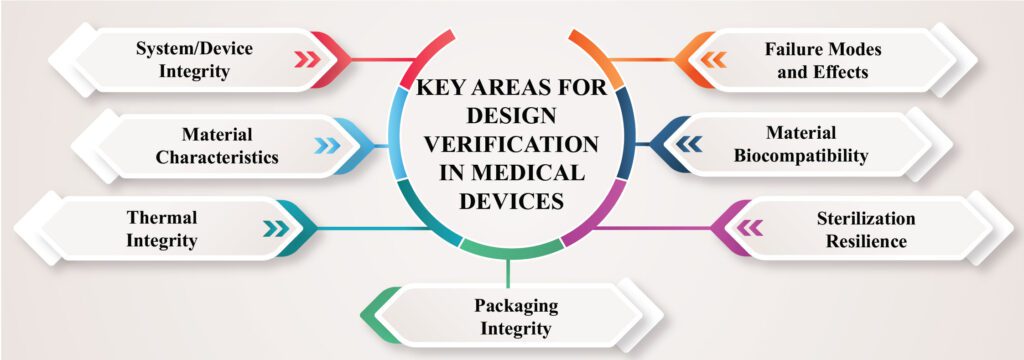
Key Areas for Design Verification in Medical Devices
Several key aspects of medical devices typically require focused design verification efforts.
- System/Device Integrity: Verification helps assess the assembled device’s ability to withstand handling and use under anticipated conditions, ensuring it functions as intended.
- Failure Modes and Effects: Testing identifies potential failure conditions, their impact on patients and caregivers, and facilitates design modifications to minimize risks.
- Material Characteristics: Verification accounts for the unique properties of materials used in the device, such as metals, ceramics, polymers, and biomaterials, which can affect its structure, durability, and performance.
- Material Biocompatibility: Verification ensures that materials in contact with patients or implanted in them for extended periods minimize the risk of adverse reactions or device rejection.
- Thermal Integrity: Verification assesses a device’s ability to dissipate heat safely, a critical consideration for energized medical devices and portable devices worn by patients.
- Sterilization Resilience: Reusable medical devices must withstand repeated sterilization processes without damage, including exposure to heat and moisture.
- Packaging Integrity: Verification ensures the packaging and packaging materials maintain sterility and integrity during handling, transportation, and long-term storage.
The Importance of Design Verification in Regulatory Approval
Design verification is not only crucial for complying with quality management system requirements but also plays a vital role in regulatory reviews. Data derived from verification activities can demonstrate compliance with specific FDA requirements, such as biocompatibility, sterilization, and electrical safety. It can also support claims of substantial equivalence with predicate devices. A robust design control process with verification and validation activities reduces the time required for regulatory review and increases the likelihood of a positive outcome.
Additional Benefits of Design Verification
Apart from regulatory compliance, design verification offers several other benefits. It helps prevent vague or misleading marketing claims by providing objective data on a device’s performance. Verified data can support more comprehensive disclosure of a device’s specific performance aspects beyond regulatory requirements. Furthermore, it allows manufacturers to differentiate their devices’ performance from competitors, offering a potential competitive advantage.
Conclusion
Claim validation studies through design verification are critical for the development of safe and effective medical devices. Compliance with quality management standards and regulatory requirements is achieved through thorough verification and validation testing. Third-party verification can further enhance the credibility of a manufacturer’s verification efforts while identifying potential gaps in the process. Claim validation studies not only ensure regulatory compliance but also contribute to the differentiation and competitiveness of medical devices in the market. By conducting comprehensive design verification, manufacturers can bring innovative and reliable medical devices to market, benefiting both patients and healthcare providers.
WorkSure® actively engages in facilitating clinical trials, in addition to supporting medical device developers through claim validation studies. With extensive experience collaborating with manufacturers worldwide, WorkSure® offers a scientific assessment to verify the accuracy of design claims. Upon successful validation and completion of clinical trials, WorkSure® presents the assessment results in a comprehensive report. This third-party verification not only helps in evaluating device quality and performance but also strengthens product marketing efforts. By choosing WorkSure®, device manufacturers can differentiate their products from those with self-declared claims, instilling confidence in buyers and patients regarding the safety and quality of the devices, while also demonstrating manufacturers’ commitment to delivering exceptional medical devices.