Post Marketing Surveillance : Ensuring Patient Safety beyond Clinical Trials
Post marketing surveillance (PMS) refers to monitoring of device/drug performance after successful completion of the clinical studies and the implementation of suitable action to improve patient safety. It is conducted after product is approved for sale by the European Union Medical Device Regulation (EUMDR) and the United States (US). It encompasses both adverse effects as well as its positive effects. The need for additional actions like changes in product labels or withdrawal of the product from the market is identified based on outcomes of the PMS studies. These changes are implemented voluntarily by the concerned pharmaceutical companies or regulatory authorities. Pharmaceutical companies, drug regulators, patients, drug surveillance organizations, specialized commentators, and consumer advocates, such as those who belong to the Society of the Drug Bulletins are the bodies involved in PMS.
The sponsors/stakeholders should use a properly designed surveillance system as the safety issue may demand device/drug withdrawal from the market, its reformulation, or recall. Appropriate resources and expertise are the pre-requisite to establishing ethical practices accompanying precise, well-balanced information in the promotional materials. Manufacturers must focus on proper documentation and submission of reports of the adverse effects to the authorities. Appropriate medical training for the staff on collecting and documenting according to the Standard Operating Procedures (SOPs) is also crucial in PMS.
Why is PMS so important?
- Benefit-risk assessment has paved the way to a systematic approach for evaluating the welfare of the available medicines. This act ensures accuracy through maintenance of a proper record of medical prescriptions and data. The robustness and systematic approach enables limited use of spontaneous reports and can address crucial safety questions.
- Sponsors often place constraints on the clinical study plan regarding the open sharing of information by clinical collaborators. A strong pharmacovigilance approval should be enforced by the legislative regulatory process after early drug release. This enhances the possibility of the peer review of the older forms of generic products already available in the market. Hence the novel therapeutic benefits of a new drug can be conquered with the existing health service. Active surveillance along with spontaneous reporting is a requisite for signal detection and stays as a cornerstone of pharmacovigilance for adherence to the regulatory guidelines.
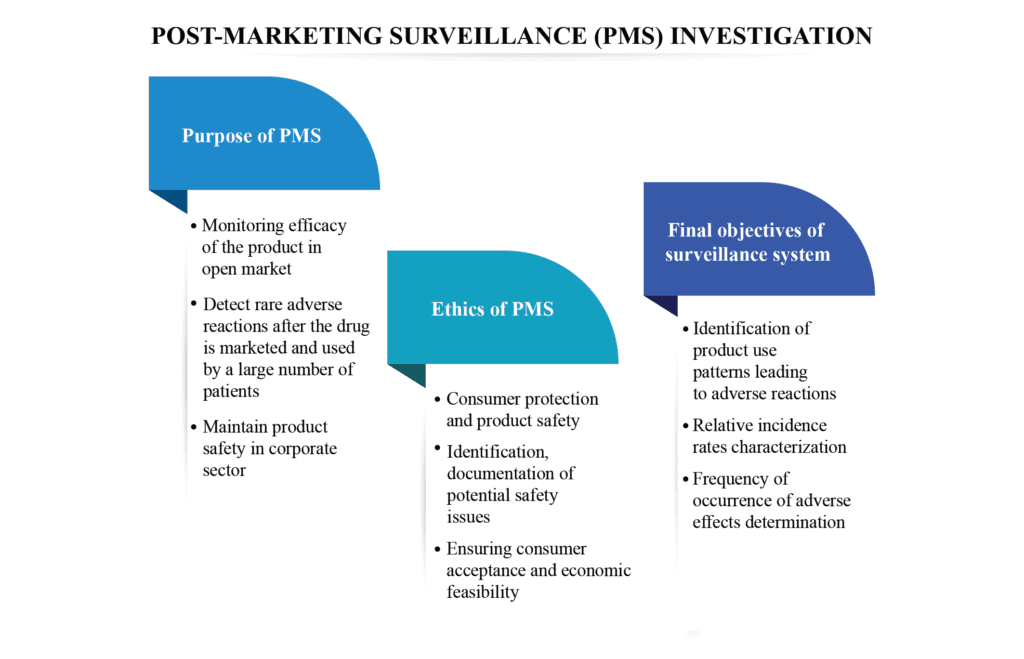
Purpose of PMS
- A PMS study is a non-interventional study to confirm the safety, tolerability, and effectiveness of a marketed drug/ device in a specific population according to the locally approved label
- Monitor the efficacy during the entire life cycle of the product in the open market
- Used to detect rare adverse reactions (that may not be known at the time of drug approval) after the drug is marketed and used by a large number of patients
- Manufacturers can implement corporate PMS to maintain product safety accomplishing the mandatory regulatory obligations
Ethics of PMS
- Consumer protection and product safety in compliance with the regulatory guidelines
- Identification and documentation of potential safety issues through monitoring and surveillance data collection of the commercialized product
- Ensuring consumer acceptance and economic feasibility
Final objectives of the post-marketing surveillance system
- Identification of product use patterns or the factors responsible for the adverse effects
- Characterization of relative incidence rates compared to market penetration, doses recommended, or other denominator data
- Determination of the frequency of adverse reactions occurring in a product, unplanned reports, cost, and the extent of consumption information
PMS Investigation at WorkSure®
WorkSure® is specialized in implementing peer services in the surveillance arena. Our highly efficient and expert team conduct proper plan for the clinical trials in compliance with the regulatory standards, assuring high-quality data. Special emphasis is given to include the results of the data generated from the PMS plans along with the corrective and preventive actions in the PMS report. Additionally, a periodic safety update report is provided to present a comprehensive update to regulatory authorities on a medical product’s global safety experience. Our Electronic Data Management System ensures top-notch results for your research and clinical trial needs. Moreover, WorkSure® guarantees PMS investigation thoroughly without any discrepancy and executes the prospective as well as retrospective study if necessary post-marketing surveillance of the product.