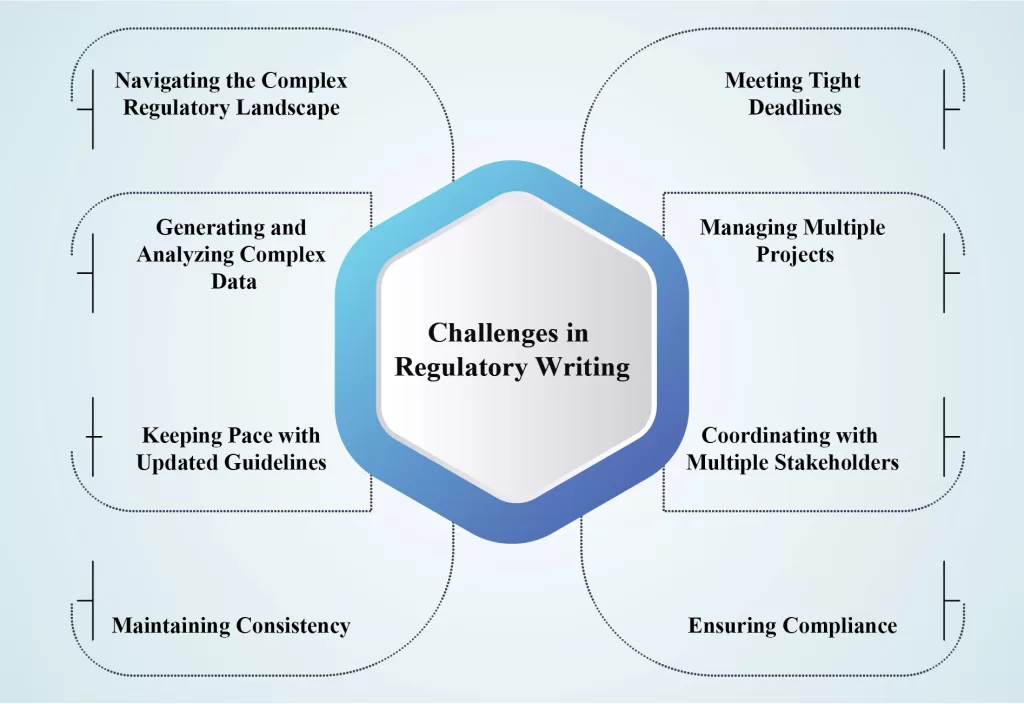
Medical writing regulatory is the development of various documents intended for submission to Health Authorities (HAs). These documents play a crucial role in conveying essential information accurately, transparently, and clearly to reviewers while adhering to relevant guidelines. Regulatory medical writers hold significant responsibility in the preparation of these documents, ensuring […]
Posts archive for December, 2023
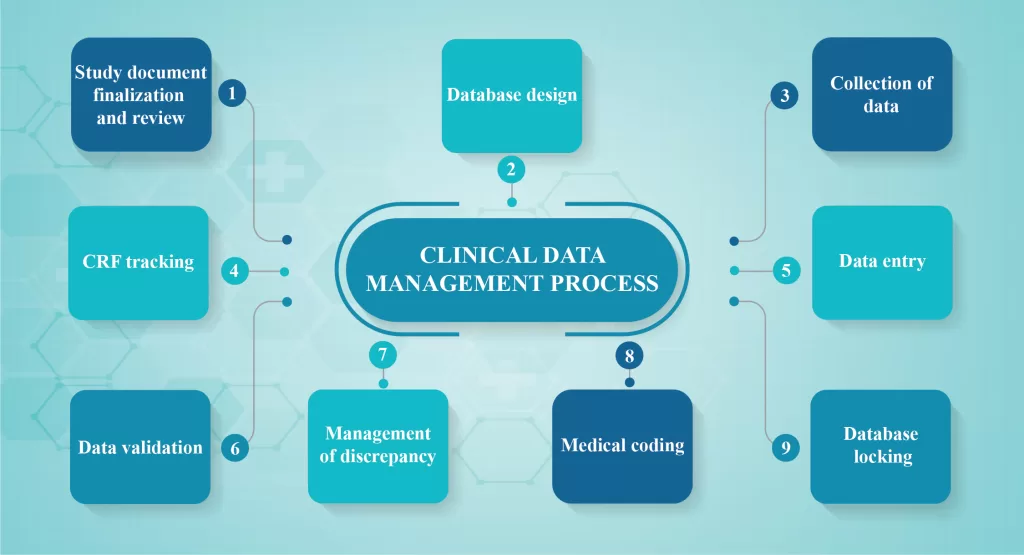
CLINICAL DATA MANAGEMENT PROCESS OVERVIEW
Clinical data management is a cyclic process of collecting, cleaning and managing data in accordance with the regulatory standards. The primary goal is to provide high-quality data obtained by reducing missing data and minimizing errors and gathering maximum data for further analysis. The data must be reliable, complete, updated and […]
Clinical Trial in India: Exploring Innovative Therapies
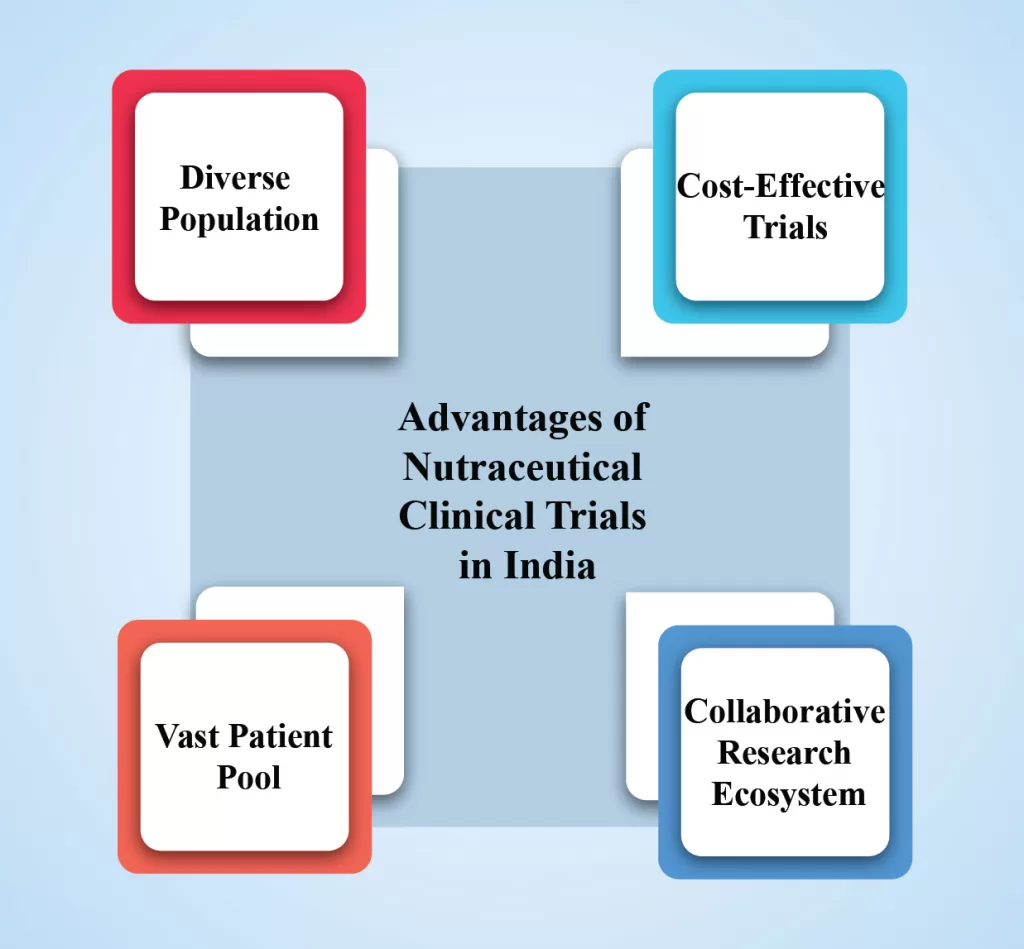
Clinical trial plays a vital role in evaluating the safety and effectiveness of nutraceutical products. Nutraceuticals, the fusion of nutrition and pharmaceuticals, are gaining popularity as natural compounds that offer health benefits and disease prevention. They also provide a crucial avenue for validating these innovative therapies, exploring their potential benefits, […]
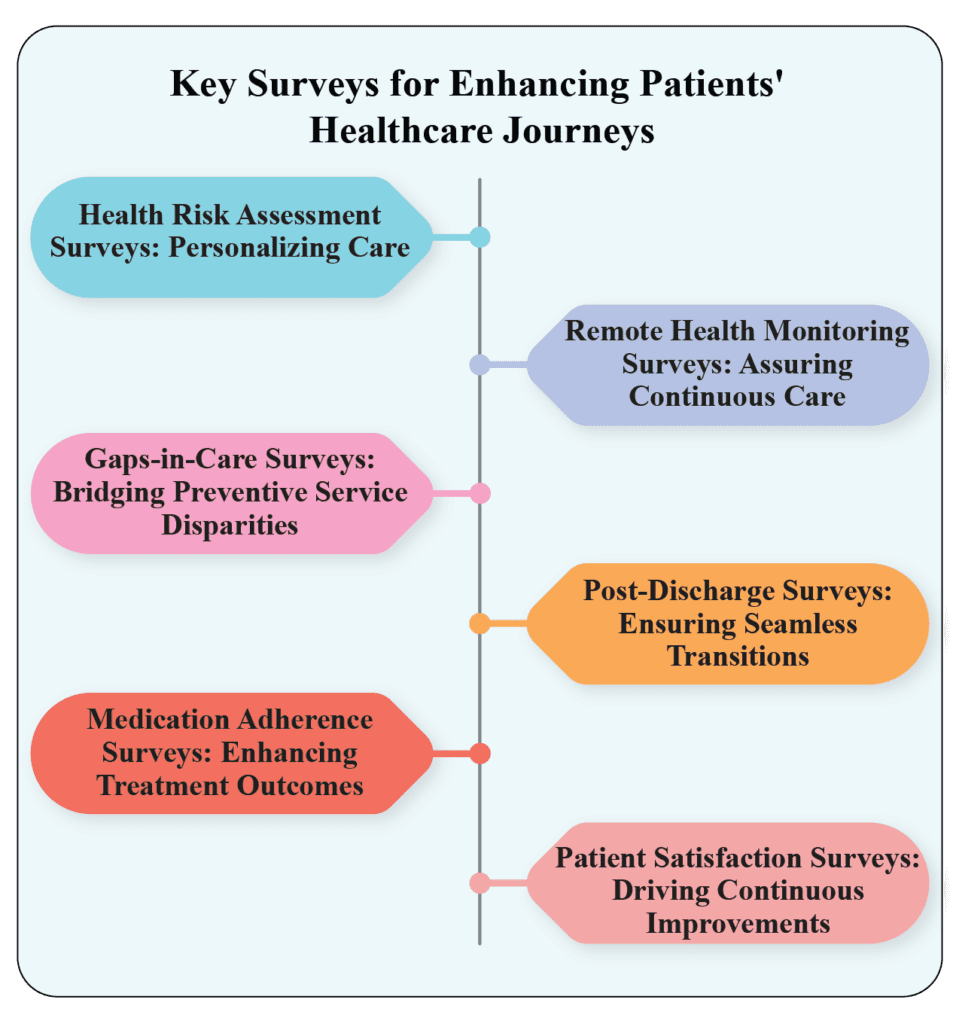
Healthcare Survey Role in Enhanced Journey: Personalized Care at its Best
Healthcare Survey : Acquiring feedback on the quality of services and care experienced by patients is an essential data asset for healthcare facilities dedicated to propelling their quality improvement initiatives. One effective approach to evaluate patients’ perceptions is through the use of health surveys. To truly transform the patient experience, […]
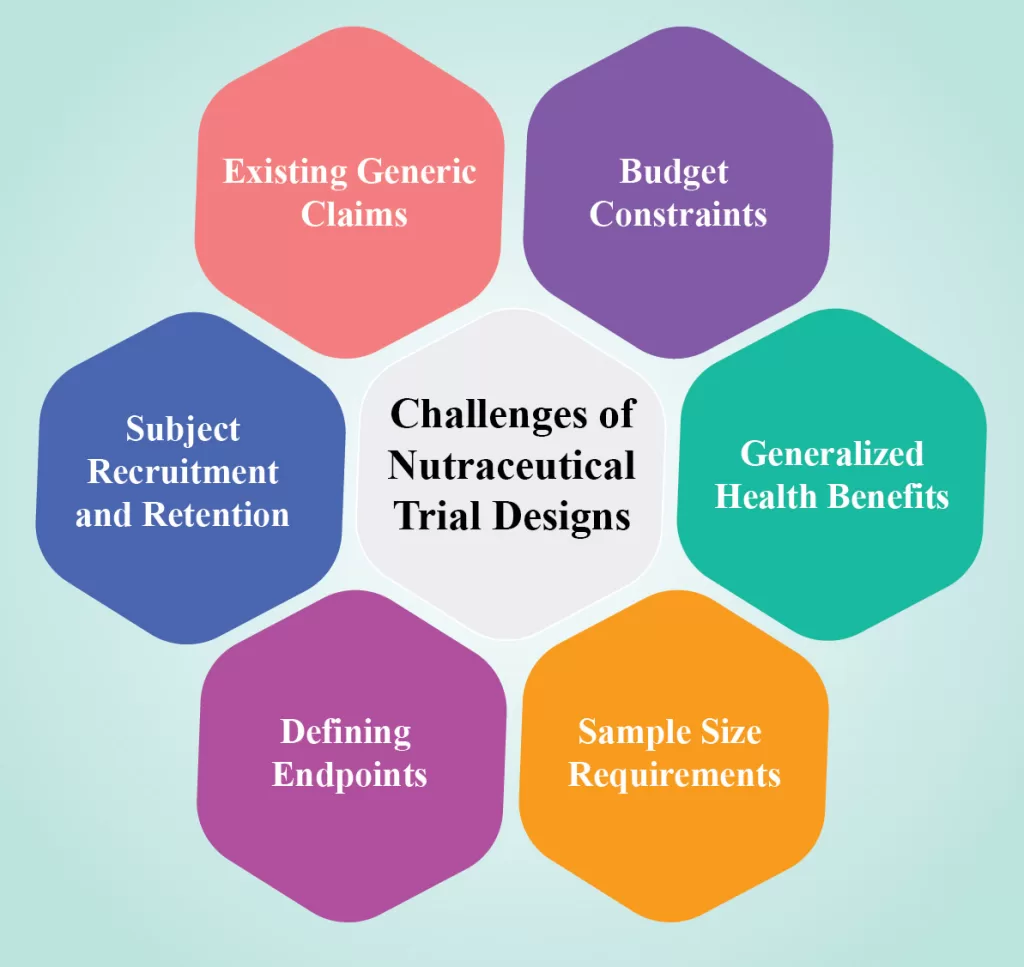
Clinical Trial in Nutraceutical World: Unveiling the Science behind Wellness
Clinical Trial in Nutraceuticals, which combine nutrition and pharmaceutical properties, have gained significant attention in promoting physical health, immunity, and longevity. A nutraceutical is any substance that is a food or part of a food that provides medical or health benefits, in addition to its basic nutritional value. These products […]
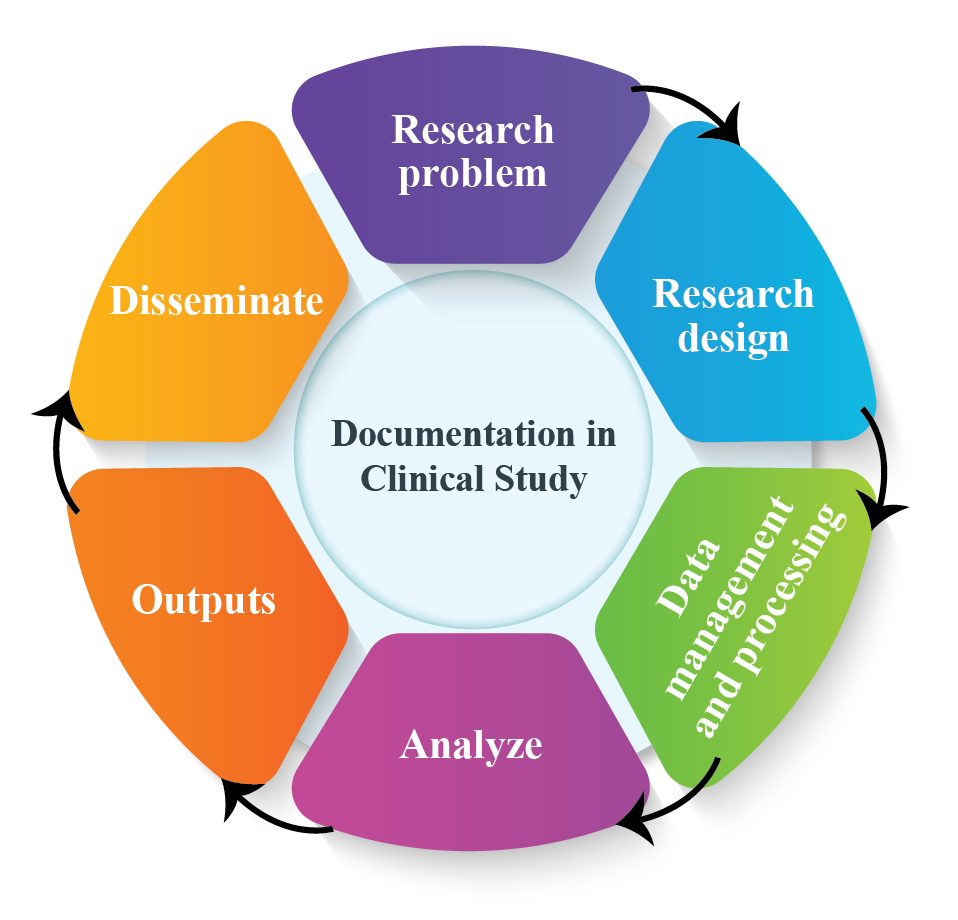
Clinical Study or Scientific Research: Importance of Documentation
Clinical Study: Documentation plays a vital role in clinical study or scientific research. It validates how authentic the research data was collected and verify the result of data. Clinical practice documentation is essential for communication among healthcare providers. It is from this documentation that protocol-specific data are abstracted from and transferred […]
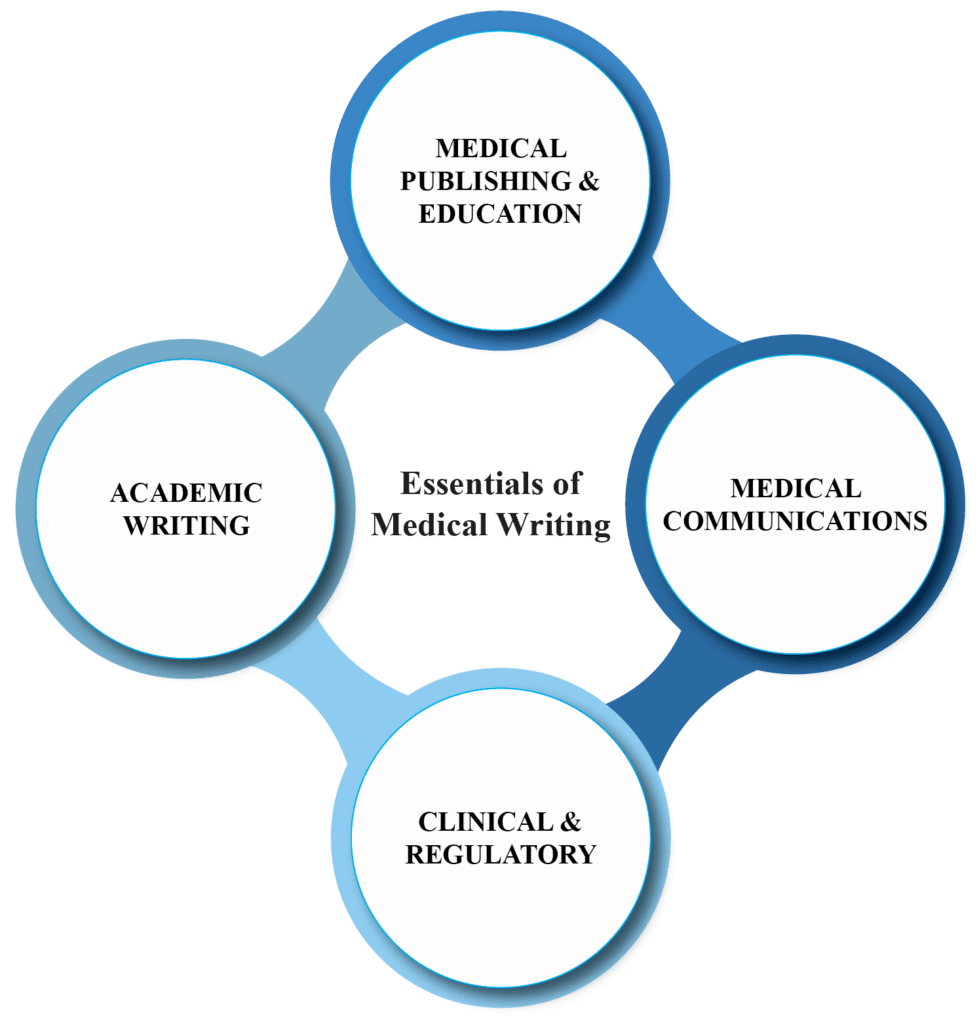
Medical Writing Essentials: Breaking down complex medical concepts
Medical writing involves writing and communicating scientific documents of different types which include regulatory and research-related documents, disease or drug-related educational and promotional literature, publication articles like journal manuscripts and abstracts, content for healthcare websites, health-related magazines or news articles in a clear, brief, plausible, absolute, and convincing manner. The […]
Recent Posts
- Regulatory Affairs in Action: Recent FDA Drug Approvals and Medical Breakthroughs
- Medical Writing Regulatory for Product Excellence: Crafting the Path to Success
- CLINICAL DATA MANAGEMENT PROCESS OVERVIEW
- Clinical Trial in India: Exploring Innovative Therapies
- Healthcare Survey Role in Enhanced Journey: Personalized Care at its Best