Post market clinical follow-up (PMCF) – An essentiality for Medical Devices
Post market clinical follow-up and Post-market surveillance (PMS) refers to the monitoring of medical devices after they have been cleared for sale and are in use by the public. One of the several types of post-marketing surveillance on the use of medical devices stipulated by the European Union Medical Device Regulation (EU MDR) is a post-market clinical follow-up (PMCF). PMCF is one of the crucial parameters for PMS which is a systematic and proactive method that gathers clinical data on the intended use of clinical devices with its performance and safety.
Why conduct PMCF?
PMCF studies are designed to identify the potential for residual risks of a clinically evaluated marked device and to collect data and gain clarity regarding the long-term clinical performance of the product. Its goal is to continuously gather clinical data on the performance and safety of the device throughout its entire lifecycle. According to EU MDR, a PMCF should identify previously unknown side effects and monitor identified side effects and contraindications.
Forms of PMCF
PMCF can be in the form of:
- clinical study
- a suitable registry
- survey of the customers
- feedback from key opinion leaders
- planned follow-up for the patients using the device
Due to several forms of PMCF and the essentiality specific to each device, understanding the right approach is a challenge for the manufacturers. Till now, there are no specific guidelines for manufacturers to select the appropriate PMCF activities. Generic and specific PMCF activities and guidance on when and how to include them in the PMCF plan are discussed below:
General PMCF activities
General PMCF activities operate to gather information producing a subjective dataset that cannot be used alone as a standard to scientifically document safety and clinical performance for continued approval as the source of the data is often non-scientific with variable data quality due to different data sources. The following are the activities of the PMCF such as:
- Accumulation of clinical experience gained, for instance, from the physicians
- Feedback from the subjects or participants, for example, the patients
- Scientific literature screening
- Other sources of clinical data such as published data on similar or equivalent predicate marketed devices. The definition is absent in the Medical Device Coordination Group guidelines.
Specific PMCF Activities
Specific PMCF activities include “higher-level” operations which produce a dataset that scientifically illustrates safety and clinical performance based on case-specific data. Such activities are:
- PMCF Studies (or observational or non-interventional clinical investigations)
- Post-Market Interventional Clinical Investigations
- Data evaluation from suitable registries
- Investigator-initiated studies
- Case cohorts
- Other subject-specific clinical data collection activities
Hence, a dataset is produced where scientific methods can be applied to document clinical performance and safety, to a standard that is accepted by the industry and clinical practice.
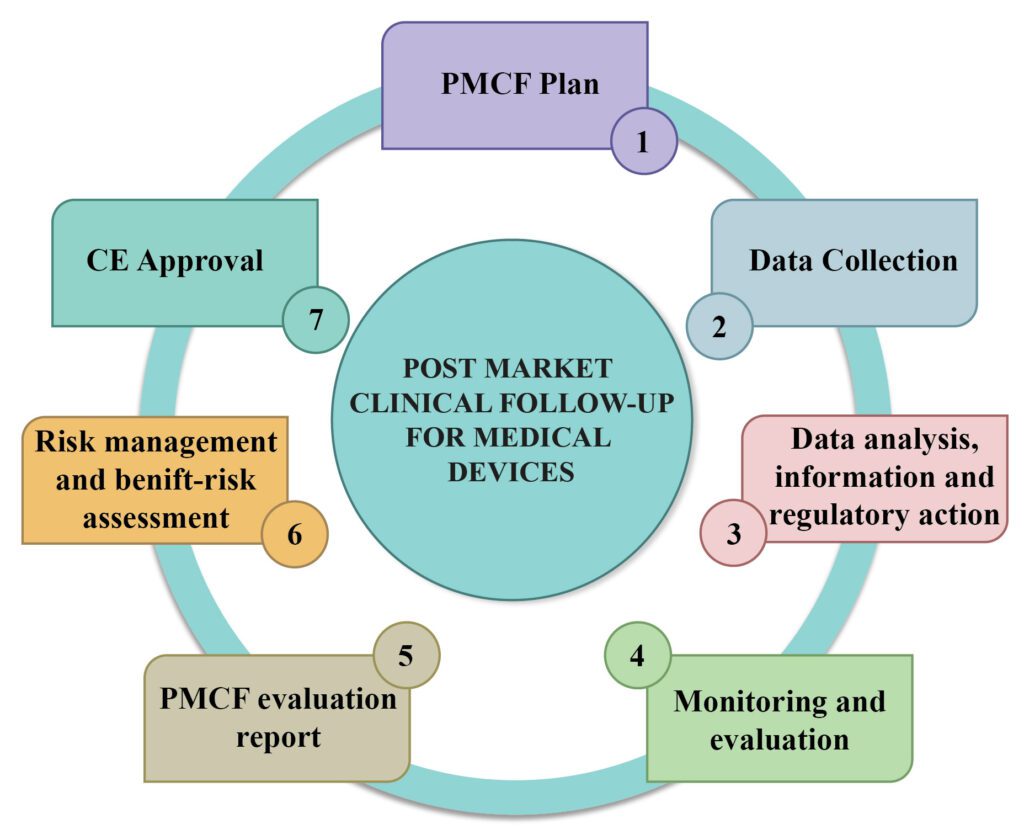
Intrinsic requirement of Post Market Clinical Follow-up plan and report
PMCF is essential for all medical devices. However, the non-application of PMCF during clinical evaluation is also justifiable emphasizing the type of the product, the intended purpose, and the associated risk class for which the PMCF is not necessary. Despite the justification, PMCF is a prerequisite and the plan then recommends a suitable place in the clinical evaluation report.
PMCF at WorkSure®
At WorkSure®, we specialize in conducting end-to-end services from study design to commercialization in conducting and documenting the post-market clinical follow-up (PMCF). Our experienced teams conduct clinical studies with an appropriate sound study design according to international standards for generating clinical data. Special focus is provided to the choice of study endpoints and the statistical basis in order to generate adequate data quality. We provide comprehensive services to support clients throughout the PMCF plan, ensure its execution with appropriate recruitment, sample and data handling, along with providing protocol planning and data analysis. Moreover, a clinical evaluation report of Post Market Clinical Follow-up with reliable results and valuable insights for the performance or safety of the medical products is guaranteed.