CLINICAL DATA MANAGEMENT PROCESS OVERVIEW
Clinical data management is a cyclic process of collecting, cleaning and managing data in accordance with the regulatory standards. The primary goal is to provide high-quality data obtained by reducing missing data and minimizing errors and gathering maximum data for further analysis. The data must be reliable, complete, updated and processed. This facilitates the use of software for audit trail maintenance and provides easy resolution and data discrepancy identification.
CDM handy tools
A clinical data management system (CDMS) comprises several software tools which may be open-sourced or commercially available for managing clinical data. In multicenter trials, the CDMS manages enormous clinical data. The most frequently used CDM tool is an electronic data management system (eDMS).
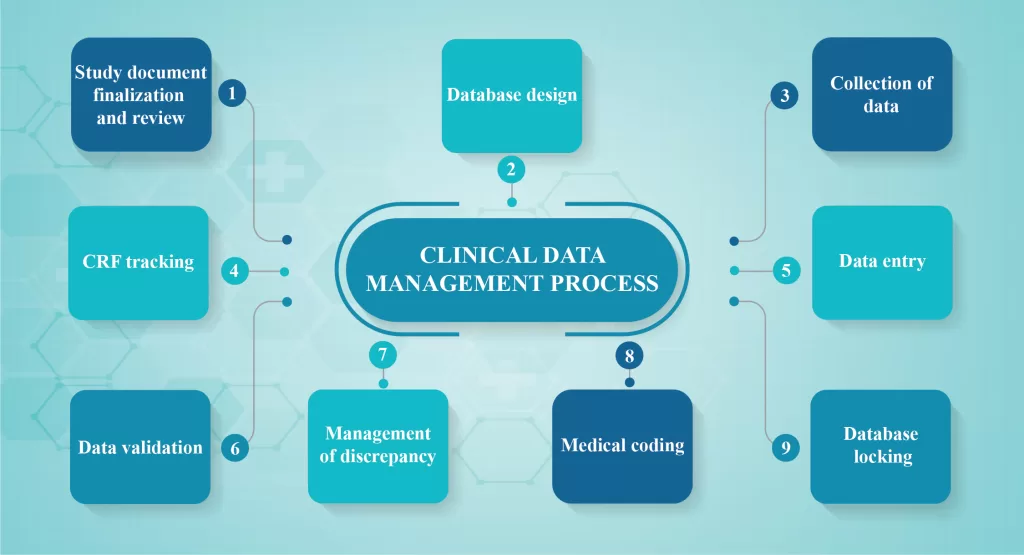
CDM process overview:
Clinical Data Management (CDM) process is initiated before the end of the clinical trial and should be error-free, valid and statistically sound. To achieve the goal, the CDM process should start before the entire study protocol is finalized. CDM should fulfill the following requirements:
- Study document finalization and review
The study protocol is often reviewed based on consistency and clarity from a database. The items essential for collection and the frequency of collection with respect to the visit schedule should be identified by the CDM personnel during the review process. The Clinical Data Management (CDM) team designs the case report form (CRF), the first step in the translation of protocol activities. The data obtained from a CRF provides valuable insights into patient outcomes and treatment effectiveness. These data should be defined with clarity and has to be consistent throughout.
- Database design
Clinical Trial Software applications are the databases built to help Clinical Data Management (CDM) task in performing several studies. Generally, these tools are adaptable and accomplish the regulatory guidelines. System validation ensures system specifications, data integrity and meets the user requirements assessed before execution. Vivid descriptions of the study encompassing the objectives, investigators, trial sites and the subject participants/ patients are defined in the database and Case Report Form structures for the basic requirements of data entry. The entry-level screens are repeatedly executed before the transfer to the original data capture.
- Collection of data
Paper or electronic versions of CRFs are filled for data collection. The most standard method is paper CRF which is further translated to the database through data entry conducted in-house. The paper CRFs are documented by the principal investigators in compliance with the regulatory guidelines for completion. The CRF is filled using the participant’s medical or other records known as the source document. The data necessary for the research are first captured in the participant’s medical or other original records (e.g., hospital medical records, x-ray reports, ECG tracings, laboratory findings, etc.) and then transcribed onto a paper or eCRF. If the data is immediately put into the CRF, it is also termed a source document. The CRF is also considered a source document if the data is directly entered into the CRF. eCRFs can be a time saver as data does not need to be entered from the paper CRF into the database.
- CRF tracking
The Clinical Research Associate monitors the CRF entries for complete review and study. These CRFs are again retrieved and submitted to the Clinical Data Management (CDM) team who tracks down the retrieved CRF and maintain the records for further use.
- Data entry
The data captured in paper CRF is entered in the centralized database. eCRF data entry requires a unique user ID, electronic signature and password. Data entry is executed strictly in compliance with the regulatory guidelines that should be prepared in addition to the data management plan.
- Data validation
Testing of data is performed following the specifications provided in the protocol. A discrepancy in the entered data is identified through edit check programs written to validate the entry. These programs are according to the logic criteria mentioned in the data validation plan. Hence, a discrepancy is defined as the data point that fails in the validation check.
- Management of discrepancy
Discrepancy management cleans the irresolvable data and collects adequate evidence for deviations observed in the data. Generally, all Clinical Data Managements (CDMs) have discrepancies in the database which are recorded and preserved after conducting audit trials.
- Medical coding
Medical dictionaries are available online for identifying and classifying the medical terminologies associated with clinical trials. Expertise in this area with proper understanding and knowledge of the e-medical dictionaries operation and the classification hierarchy is a requisite.
- Database locking
This is the final step conducted post-quality assurance. SAS databases are finalized with the statistician and CDM is closed before database locking.
Clinical Data Management (CDM) at WorkSure®
At WorkSure®, we have a highly, experienced robust team efficiently employed in the development of data management and validation plan, CRF screen design, development of the online and offline database, CRF tracking and annotation, data collection, entry, validation, management of discrepancy/query, medical coding and data reconciliation, database locking as well as unlocking. Moreover, the team is engaged in data extraction and archival, access control and data security along with data analysis.