Medical Affairs Management Strategies – Roadmap for Success
Pharmaceutical companies play a major role in Medical Affairs (MA). Medical Affairs Management team exhibits value-added leadership through their annual strategic process. The medical plan provides a pathway or the roadmap for the core medical strategy positioning all multifunctional MA competencies and common objectives. The objective is aligned with a focused medical strategy. This strategy is the standpoint for the year ahead providing a roadmap guiding to overcome challenges, take advantage of opportunities and fill scientific or clinical gaps.
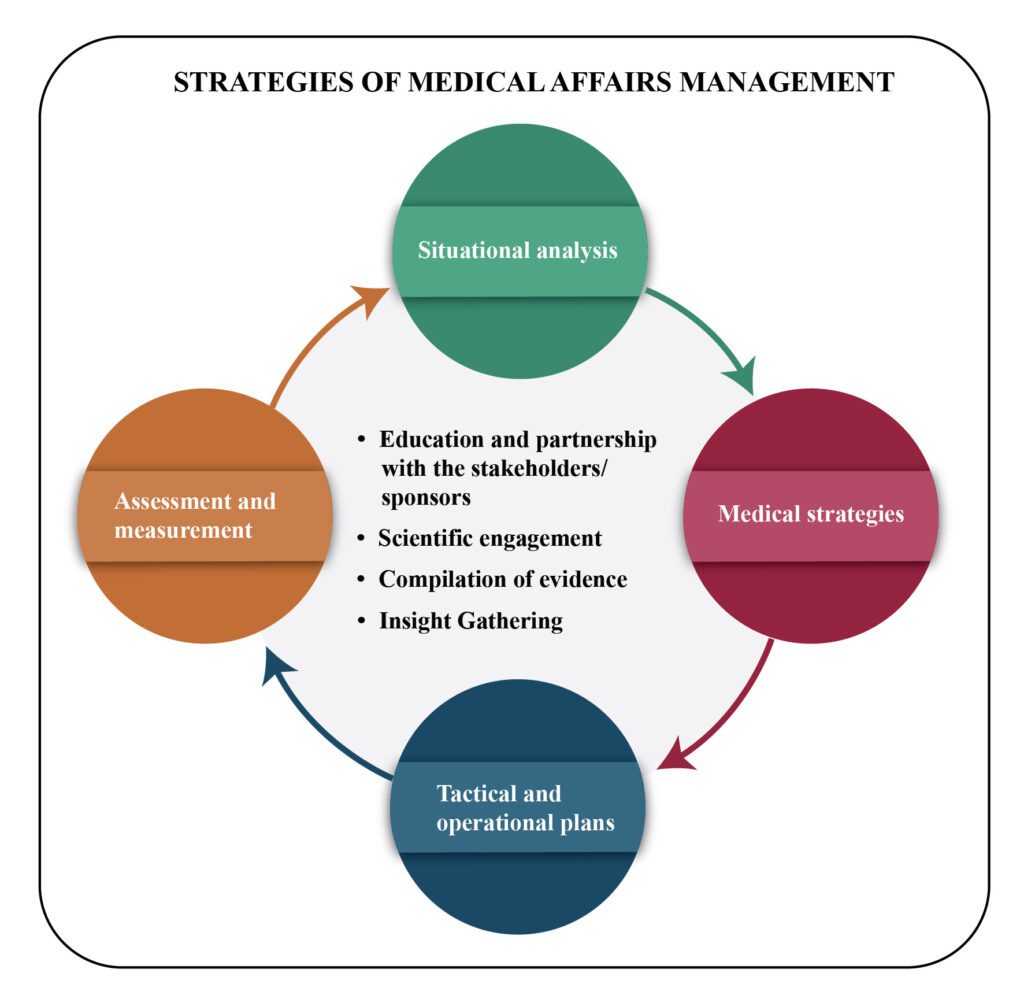
Strategic medical plan of the Medical Affairs team
MA’s strategic plan includes both situational analyses as well as medical strategies in its intellectual components. Moreover, it encompasses tactical components such as tactical and operational plans and assessment and measurement metrics. A medical plan is crucial in providing proper guidance for decision-making throughout the organization to provide an overview of the MA efforts and their impact.
Four important strategies of the MA team
MA team is concerned with the following responsibilities:
- Education of both internal and external stakeholders/sponsors on scientific topics, care for the patients, and the outcomes
- Engagement with external stakeholders for scientific discussions
- Involvement in planning and evidence generation
- Responsible for thorough understanding, collection, and compilation of the report
- Education and Partnership with the Stakeholders/ Sponsors
The MA team has to share knowledge to the stakeholders throughout the product lifecycle phases.
Product early-development phase: Build external expert interest
Pre-launch phase: Build awareness and confirm product familiarity
Launch phase: Clinical evidence and product use education
Post-launch Phase: Pursuance of medical education
Increased complexity of products and portfolio growth continually require taking steps accordingly and continuing medical education within the budgets.
- Scientific Engagement
MA should be ready to respond to market trends such as:
- Physicians are interested to be associated and interact engaged in valuable healthy scientific discussions
- Enhanced pressure to build promotional compliance while restoring transparency
- Building emphasis on scientific discussions
MA Management team is capable to manage adverse circumstances by integrating dissimilar data and responding to the requirements of the customers. Real-world evidence and scientific analysis have to be thoroughly optimized.
- Compilation of Evidence
Evidence-based decisions are very crucial. Clinical and medical evidence within the organization is presented to the stakeholders. The MA Management team communicates the company’s value in a well-balanced and evidence-based manner.
Medical affairs should efficiently consider various elements and follow an integrative approach to evidence planning and its compilation. This ownership of evidence planning and generation benefits the scientific circle, the pharmaceutical industry, the subject participants, and all other relevant stakeholders.
- An integrated approach is the pathway for the provision of better evidence to the providers and patients.
- All evidence is checked properly and the decision maker’s demands are fulfilled.
- The subject participants or the patients will experience better value-based outcomes in the form of real world benefits, and improvised health benefits.
- Insight Gathering
An insight collection process helps in the insight gathering, documentation, reporting, and follow- up. The insight compilation should achieve to close internal data or knowledge gaps.
Conclusion
Medical Affairs Management should follow the roadmap of the clinical plan following the strategy of educating the stakeholders or the sponsors on the scientific evidence and providing detailed data collection and report insight.
WorkSure® enables you to develop a stronger, strategic, high-performing team by positioning your strategy to the requirements of different stakeholders. We have a strong Medical Affairs team who will guide you to investigate several market demands. Proper planning and highly reliable quality data are ensured for the manufacturers and the publishers where they can investigate and invest wisely in research and development. Physicians can make independent decisions about innovative treatment alternatives. WorkSure® emphasizes providing a better understanding to the patients about the latest therapeutic advancements and patient outcomes.